Embryology - Biology 104, Spring 2006 - Albert Harris and Corey Johnson
LECTURE - March 20, 2006, by Albert Harris
-
Beloussov, Lev (2006). An interview with Albert Harris. Direct physical
formation of anatomical structures by cell traction forces.
Int. J. Dev. Biol. 50: 93-101.
What to learn about the contents of the lecture on March 20:
Although this lecture summarized the history of some research and a theory that was proposed by one of your Instructors, please do not feel any pressure to believe that this theory is necessarily correct. What you should learn are the following.The protein called collagen is the major extracellular protein of human and animal bodies. Collagen is a large protein, that many kinds of cells secrete. Each molecule of type I collagen is long and stiff (it's a triple helix, of a different kind than the alpha helix!) and these molecules tend to form chemical bonds to each other side-by side.
There are more than a dozen different kinds of collagen (called type I collagen, type II collagen, type III collagen, type IV collagen, etc. up to about XVIII, and more may be discovered)
Somewhat to everyone's surprise, it has turned out that many different anatomical structures are made largely of type I collagen, which must be 90%+ of the collagen in our body, or maybe 99%. Tendons are almost entirely type I collagen, bone is 33% type I collagen (and 67% calcium salts), organ capsules (including the visible white part of your eyeball) are mostly type I collagen, and so are several other parts of your body, including the walls of arteries and veins.
Most scientists had somewhat expected that structures that are geometrically so much different from one another would probably be made of different kinds of collagen, so that the differences in molecular properties would cause the individual molecules to self-organize to form these different kinds of structures. So it was a paradox that it didn't turn out that way. On the other hand, cartilage is made largely of type II collagen, so there definitely are examples where different kinds of collagen do form different kinds of anatomical structures.
A. Harris began studying force-exertion by individual tissue culture cells in Cambridge in 1971, using clotted fibrin (the protein in your blood that forms the structure of blood and lymph clots, and also scabs). The first invention of tissue culture by Ross Harrison depended on using clotted fibrin from frog lymph as the material for the cells to attach to, although you don't have to learn this last point. Nor do you need to know that I was taught how to do tissue culture in graduate school by the lady who had been Ross Harrison's life-long technician and assistant.
Since at least the 1930s, everyone believed Weiss' theory that cells distort fibrin clots biochemically, by causing them to shrink locally. I was taught this as a proven fact, and believed it completely until Michael Abercrombie suggested to me that as cells crawl they must be exerting some force, and that force might be the real cause of the apparent "shrinkage" of clotted fibrin. Then I immediately began trying to develop that idea into some kind of experimental method to measure the strengths and the directions of the forces exerted by individual cells. After many failures, I invented a (very simple!) method for cross linking certain kinds of silicone fluids to form microscopically-thin layers of rubber, thin enough that individual cells could wrinkle it by the "traction" forces that they used for crawling.
This silicone rubber method finally convinced everybody that cells do exert traction, and other researchers have since developed the method further into a technique that they call "Traction Force Microscopy", and millions of dollars of research money are spent on this, none of which I get, for some reason.
Anyway, my graduate student David Stopak, around 1980, began studying the exertion of cell traction of gels made of type I collagen. He dissolved this collagen from the tendons of rat tails. When you do scientific research, you wind up doing some strange things! Among his observations was that clumps of mesenchymal cells from chicken embryos, when put on or within gels made by reprecipitating this rat tail tendon collagen, would cause the collagen molecules to line up for long distances, as far as all the way across Petri dishes!
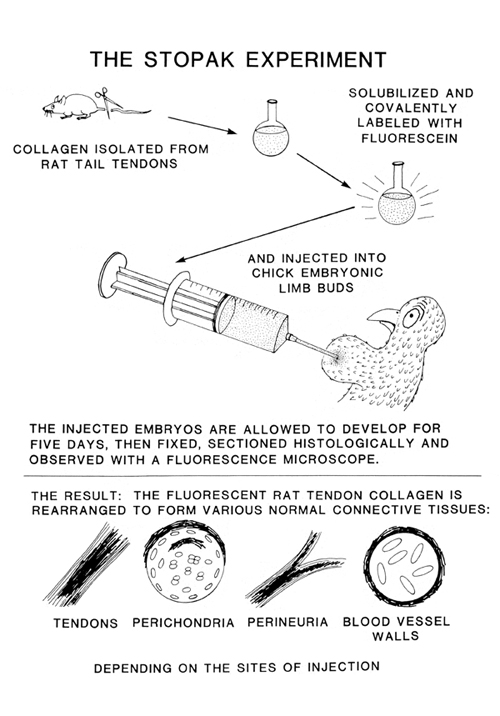
Based on this and many other experiments and unexpected observations, he and I independently concluded that maybe some kinds of cells use traction not just to crawl, but also to line up collagen, and sometimes to pack into compressed sheets, and other patterns. When David got his PhD for this research, he then went to Stanford to be a post-doctoral fellow (like I had been a "post-doc" at Cambridge, in the other direction). David's main post-doctoral research consisted of chemically reacting isolated type I collagen with Fluorescein, a chemical that glows yellow when you shine UV light on it. After making collagen fluorescent in this way, he injected it into living, developing chicken embryos. We hoped that maybe this injected collagen would get rearranged to form some of the geometric patterns that we had observed that cell traction will produce in tissue culture. The results were better than we had hoped, and we published them in one of the most competitive and well-respected journals, in which much previous research on collagen had been published.
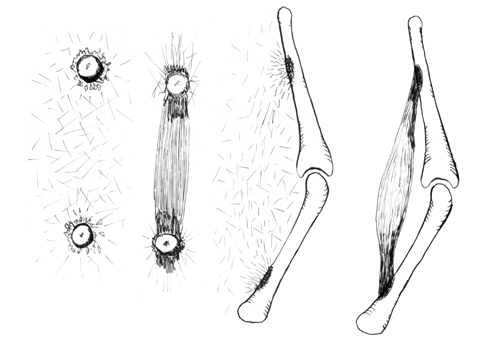
Diagram comparing the experimental observations and the hypothetical proposals of Stopak and Harris
Unfortunately, none of the experts on collagen were convinced, and some of them became very critical of my whole research program (and the horse it rode in on, as the saying goes). They refused to mention any of our research in their own papers, text-books etc., with very few exceptions. Our research was more accepted in Europe than in the US.
What you should learn is that type I collagen somehow becomes arranged into many different geometric patterns in the body, and nobody knows for sure what mechanism are used to cause these arrangements. Jerome Gross of Harvard Med. School advocates the theory that variations in pH or other local chemical properties causes collagen to self assemble into different patterns, and he has shown experimentally that this can occur in test tubes. The main alternative theory is that cells secrete collagen as they crawl along, like a spider making its web. Evidence for this includes electron microscope sections that clearly show mesenchymal cells that are secreting collagen in the right places, times, and directions, that would be needed to form the different patterns. So those, or some other mechanism that no one has invented yet, may be the real mechanism that causes collagen patterns. More research is needed.
The most tantalizing phenomenon in this field is the formation of perpendicular layers of collagen, alternating directions by 90 degrees, like lumber stacked to dry. In the most anterior structure of our eyes, there are about a hundred layers of type I collagen, arranged in alternating perpendicular layers. There are other examples in salamander dermis, in the sheath of the notochord, and the cortex of intervertebral disks in your spinal cord. There are also a few examples in which muscle cells get lined up in a series of alternating perpendicular layers. Mammal tongues have this structure. Nobody has ever been able to figure out what mechanism causes one layer of collagen to be oriented exactly perpendicular to the adjacent layers, nor what could control such a perpendicular arrangement of muscle cells in the tongue, or other places.
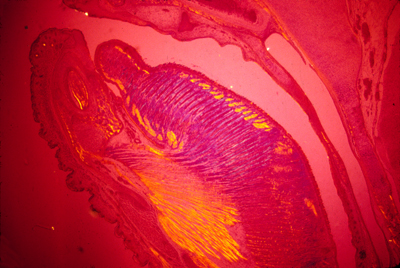
Photograph of a cross section through a mouse tongue, showing alternating perpendicular layers of muscles. Polarized light was used to create different colors where the fibers are oriented in different directions.
Maybe you can figure out one or more possible mechanisms that could produce such a result, and also invent some experiments to try to prove that your mechanism is how it really works.