Please notice how analogous this is to what happens when you separate the first two cells of embryos of regulative species; also notice the similarity to what happens when you split early limb buds.
Actually, these edges of the lateral plate mesoderm can form more than two hearts, when this tissue is cut into pieces. As many as nine separate hearts were formed by one chicken embryo in some experiments done by French embryologists.
The heart has to start pumping blood very early in development, long before the nervous system or skeletal muscles begin to function. This is because oxygen has to be carried to the tissues, and CO2 carried away. The heart begins to beat even before the kidneys start functioning. The heart starts as a simple peristaltic tube, with no valves and no separate chambers; it then expands and gradually changes shape, subdividing into two chambers and then into four chambers, with 4 sets of one-way valves. This increased complexity occurs by modification and addition to the same original tissue. Please notice that these shape changes all occur while the heart is beating! (Have you ever tried to put on a sweater while driving a car? Don't try it!) The heart starts as a simple tube, which then gets twisted back on itself, constricted into atria and ventricles, and then partitioned into right and left (a contractile sheet of tissue squeezes right down the middle!) Before birth, blood does not need to be pumped through the lungs, and the following system for by-passing the lungs exists: There is a "trap door" opening between the right and left atria called the foramen ovale, and also a short artery named the ductus arteriosus, which connects the pulmonary artery to the aorta.
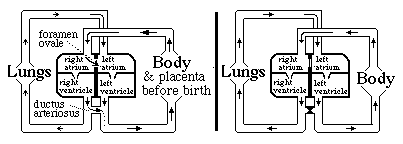
Without the ductus arteriosus, blood pumped out of the right ventricle would have no choice but to go to the lungs. And without the foramen ovale, ONLY blood returning from the lungs would reach the left atrium.
Questions to think about and discuss: a) Which direction does blood flow through the foramen ovale, prior to the moment of birth? b) Which direction does blood flow through the ductus arteriosus before birth? c) When the lungs expand and fill with air, that reduces the mechanical resistance to blood flowing through the capillaries: How should eventually that change the relative pressures in the left versus the right atrium? d) What about the relative amounts of pressure in the aorta versus the pulmonary artery, before and after the lungs expand at birth. e) Why do you suppose that the thickness and strength of the cardiac muscle walls of the right and left ventricles are the same before birth...? but after birth gradually become about four-times thicker and stronger on the left side than the right side? f) If higher oxygen levels in the blood stimulate constriction and closing of the ductus arteriosus at the time of birth, what can that accomplish?
Normally (in mammals) the tissues fuse together in both the ductus arteriosus and the foramen ovale, closing both permanently. In as many as 25% of people, some small hole remains between the atria. (without any symptoms) Many kinds of serious birth defects in human hearts result from improper formation of the interventricular septum (septal defects). Sometimes the ventricles remain connected; sometimes the aorta or the pulmonary artery are connected to the WRONG ventricle! Question g) Do you see how such "blue babies" can develop up to the time of birth, more or less "normally", but then have severe problems?
The special kind of epithelial cells that line all blood vessels are called endothelial cells.
The narrowest blood vessels are called capillaries, and their walls are made of endothelial cells only (one cell layer thick; and with few or no smooth muscle cells of fibroblasts to provide mechanical reinforcement or control constriction. Arteries are made of layers of smooth muscle cells (and some fibroblasts) and type I collagen fibers wrapped around their capillary cells. Veins have fibroblasts and collagen wrapping the endothelial tube that lines where the blood cells are. Not only are artery walls much thicker than those of veins, another difference is that artery walls are strongly contractile in all but the largest arteries (like the aorta). Notice that the blood pressure in capillaries is often almost as large as the blood pressure in arteries! How are the thin walls of arteries able to withstand such strong pressure? And if capillaries (made only of endothelial cells) are able to withstand so much pressure, then why do the walls of arteries need thick layers of smooth muscle cells and collagen fibers? The answer is that Pressure=Curvature*Tension. The high curvature of the capillary walls enables them to hold back very strong pressures with small amounts of tension This is one of many examples where you need to know about P=C*T in order to make sense of important embryological and anatomical phenomena. Few textbooks even explain the definition of curvature, in a mechanical and mathematical sense. This same physical principle is why hydraulic equipment like back-hoes can be powered by high pressure fluids driven through narrow rubber pipes.
The first beginnings of the vertebrate circulatory system are a series of unconnected endothelial sacks, called blood islands, with red blood cells and white blood cells differentiating inside; most of these are in the yolk sac. Their endothelial cells crawl and connect to each other to form hollow tubes: the first capillaries. Only much later does bone marrow become the place where blood formation occurs; early embryos don't have bones; and when the skeleton does form it is nearly all made out of cartilage, which is solid, without a marrow, and isn't even penetrated by blood vessels (i.e. NOT vascularized) which is what you call it when these endothelial cells penetrate a tissue and form a system of capillaries, and later arteries & veins. Early embryos do not have any bone marrow yet, because nearly all their "bones" are still made of cartilage, and are solid inside. The yolk sac is one of several different organs that are used as locations for the hemopoietic stem cells which form red blood cells (and also white blood cells). These stem cells move to the liver, and later to the bone marrow. Embryonic and fetal hemoglobins are different from (and coded for by different genes than) the hemoglobin we have in the red blood cells we make after birth. Fetal hemoglobin binds oxygen slightly more strongly than adult hemoglobin, which increases the amount of oxygen that is transferred across the placenta from the blood of a pregnant woman to the blood of the fetus developing inside her. Two other interesting aspects of this phenomenon are #1) that the genes for embryonic and fetal hemoglobin proteins are directly adjacent to the genes for adult hemoglobins, with the early blood cells activating one gene, and then later blood cells activating the gene next to that one. The mechanism isn't known, nor is it known whether there is any similarity to the mechanisms that cause adjacent Hox genes to be transcribed in adjacent tissues. #2) A few people continue to make fetal hemoglobin all their life, instead of switching to the adult hemoglobin genes, and the symptoms of this are almost unnoticeable! So far as I know, it hasn't yet been discovered what mechanism causes this; but it could become a life-saving treatment for victims of sickle-cell anemia (and other genetic defects of adult hemoglobins), if you could discover a drug or other treatment that would switch their hemopoietic stem cells back to transcribing the genes for fetal hemoglobin.
The geometry of the early vertebrate circulatory system is organized much like that of our distant fish ancestors, with blood being pumped front-ward out of the heart and around the sides of the neck in a series of 6 paired arteries like those used to provide blood to the 6 pairs of gills in a fish. Even birds and mammals form these aortic arches, even though there are no actual gills; they are in the places where gills would be. In mammals, the fourth left aortic arch becomes the aorta..