Embryology Biology 441 Spring 2010 Albert Harris
Lecture Notes for March 29
What embryology has learned from fly genetics:
Revolutionary (and unexpected) discoveries came from systematic searches for genes that control early development. The biggest surprise has been that very similar sets of genes also occur in vertebrates, and almost all multi-cellular animals.The base sequences of these genes are so similar that they must have common evolutionary origins. (too similar to be convergent evolution, especially when they have different effects or functions.)
Many people conclude that the homology of genes proves that all animals must use the same basic mechanisms to create anatomical patterns as flies do, ignoring big differences such as mosaic vs. regulative development.
This is not too logical: If the genes had been discovered first in mammals, That wouldn't be evidence that fly development is really more like mammals. We will carefully consider both differences and similarities. Some have looked for evidence of mosaic development (early cytoplasmic detriments in mouse oocytes, like there are in flies). A paper claiming this from the university of Missouri turned out to have photographs faked using Photoshop, & was retracted. when scientists strongly believe that something ought to exist, sometimes they either see what isn't there, concentrate or rare events or artifacts, or pressure students to distort their results. But fraud is rare in science, and quickly caught. In this Missouri case, referees of the manuscript spotted details in the photos, and the editors asked other scientists to repeat the experiments
Among the bigger surprises was that the "same" genes control where eyes form
In flies as in vertebrates, even though the structures of the eyes evolved separately.
(compound eyes versus camera eyes!!)
Non-homologous organs having their locations controlled by homologous genes.
__________________________________________________________________
If mutating a certain gene causes abnormal development of a certain organ, then we conclude that the function of the protein coded by this gene must be part of the control system by which that organ is built.
A systematic searches for as many different mutations as can be found that change a particular organ or function is called a genetic screen. Genetic screens have become a central part of modern embryology.
Sometimes, researchers just have to look at thousands or millions of animals to find the 2 or 3 or a dozen that are abnormal in a particular way. ("needles in a haystack")
Other times, it can be arranged for the special organisms to find themselves.
For example, if you were looking for mutations with defective phototaxis, you could plate out a few million cells, and then shine a light from one side. 99.9% of the cells would crawl toward the light; but the mutants wouldn't. The cells left behind, or moving randomly, would have a high percentage of mutants of the kind you want (plus mutants with defective locomotion).
A good screen will save you 99% + of the effort.
Some biologists' reputations (and professorships) have been earned largely by inventing a new genetic screen. Many are fiendishly clever!!
It is a huge stimulus to your cleverness to search for mutants, one by one! You get a lot of time to think of better & more powerful screens.
When you hear geneticists talking about "screens", it's probably not wire mesh.
____________________________________________________________________
With a good screen, plus a lot of effort, one can hope to identify ALL the different proteins that participate in some process, or development of some organ!
For about the last 15 years, a large percentage of researchers follow this approach.
Even when a "knock out" is an eventual part of proving the function of a gene & protein, there almost always had to be a screen to find the mutation in the first place! Elementary textbooks and courses tend to mislead about this, because it' so easy to visualize eliminating a gene (which is really done by inserting a big block of DNA into the middle of it) and then seeing the effect.
It's as if a history book said that X was discovered to be a spy because
after his execution not so many secrets reached an enemy.
That wouldn't have been the original evidence.
_____________________________________________________________________
Drosophila have key advantages:
1) Small size (& not cute enough for PETA to care how many you kill)
2) Transparency and accessibility of embryos and larvae
(so you can see anatomical abnormalities without killing either mother or embryos)
3) Rapid life cycle (if one organism has a new generation every week, and another organism has one generation per year, then genetic research will progress 52 times as fast using the organism that breeds a week after its own fertilization
4) Small genome (~5,000 genes) (although we have scarcely twice that)
5) Genes are not redundant. (Not tetraploid, like Xenopus)
- One set of proteins seldom can substitute for another;
Such ability to substitute has been a big unforeseen problem with vertebrates!
Experimenters "knock out" some gene of central importance, but
There is little or no effect on the mutant animals.
{Forgive me for digressing about comparable examples: the development of monoclonal antibodies would have failed unless newly stimulated lymphocytes fuse
with other lymphocytes much, MUCH more frequently than mature lymphocyte plasma cells fuse with each other in response to the same treatments. Who knew? Who suspected? If new & old lymphocytes both fused equally well, then monoclonal methods wouldn't automatically enrich for lymphocytes specific for whatever antigens the sacrificed mouse had recently been exposed to.
History of science is interesting, but seldom notices "luck" of these kinds, when methods work unexpectedly well for reasons not noticed until later.
In my own case, the main reason I didn't fail to develop elastic substrata for studying cell locomotion is that the real functions of fibroblast traction are mass rearrangement of collagen, and wound constriction! Only later could I increase the sensitivity enough to study locomotion itself.
__________________________________________________________________
Chapter 8 in Slack's book is the single best description I have ever seen of the methods and results by which "the breakthrough" in fly embryology was achieved.
BUT DO NOT BE FRIGHTENED BY ALL THE DETAILS. I will provide a review sheet stating specifically which parts you will need on exams.
NEVERTHELESS, PLEASE READ THIS CHAPTER CAREFULLY,
including pages 222 through 266, which may seem to go on forever,
and which include difficult concepts, methods and conlusions.
Consider reading it out loud to yourself or into a recorder.
Swim through all those complex details, and get a feel for their strangeness.
This stuff IS "rocket science".
In brief: Geneticists developed several fiendishly clever tricks by which to "recover" recessive lethal mutations.
The first key idea is that those genes that code for proteins that control early stages of embryonic development are especially likely to be lethal when mutated.
So how do you breed dead flies?
That's where the fiendish clever genetic tricks come in.
Even harder, how do you screen for lethals that die with consistent anatomical (geometrical) abnormalities.
You want genes whose mutation produces two tails and no head,
Or that produce a head directly connected to a tail, with no middle,
Or just the anatomical structures of the middle,
Or all back and no stomach.
Or an extra pair of legs where the antennae should be,
Or two extra wings
Or extra eyes to develop from cells that should have become part of the tail.
Christine Nusslein-Volhard (a West German woman scientist) and Eric Weischaus (a Yale Biology PhD) and several others spent ~10 years finding 140 such genes Seventy three such genes and the effects of mutating them are listed starting on page 267 of Slacks book.
I will give you a list of a few of these to memorize, but please read them all.
Incidentally, the great majority of these genes code for transcription factors.
In situ hybridization was then used to map the times & locations in developing embryos where these various genes get transcribed?
What result would you guess? If organ X fails to form as a result of mutating gene Y, then don't be surprised if messenger RNA of the normal Y gene appears in stained embryos at higher concentrations at the normal location of organ X. That's the pattern!
If your in situ stain gives that sort of result: submit a paper about it to Nature.
If you get a different result, try to make sense of it, or submit it to a lesser journal.
Mostly, results have fallen into these patterns, plus genes with very similar Base sequences were then discovered in mammals, and worms, etc Even though mutating the mammal homologs produces different phenotypes Embryology is never going to be the same! It is morphing into a game of figuring out how to explain normal development is accomplished by particular combinations of normal (wild-type) alleles of these genes.
For example, the gene-that-when-mutated-causes-two-tails-and-no-heads (Bicoid) gets transcribed in the fly's mother's cell ("maternal effect"!), gets put in the front end of the fly embryos, and its translation there results in a diffusion gradient through syncytial cytoplasm that activates transcription of a series of other genes for transcription factors, different ones depending on its concentration (making it a morphogen, in Lewis Wolpert's sense of that word, and fitting theories that go back a century), and then those transcription factors bind to DNA and selectively stimulate other genes, according to which combinations of other transcription factors are present at each location (a combinatorial alternative category of morphogen!) [which some other people had hypothesized]
One family of transcription factors controls where and when transcription will be stimulated in the next (of five) families of transcription factors will be activated, much like dominos knocking over dominos, and somewhat like a cuckoo clock, designed by a cuckoo clockmaker.
But it's all true.
But maybe some aspects of development are invisible to this approach?
===========================================================
SOME MAJOR DIFFERENCES IN INSECT EMBRYOLOGY (among which fly development is somewhat special)
1) All 3 axes (anterior-posterior, dorso-ventral, medio-lateral) are already irreversibly determined before fertilization by signals and m-RNA from surrounding maternal tissue.
Notice the contrast to vertebrates, especially mammals! Not to be a spoil-sport, but what are we supposed to think when the Wnt gene or some such turns out to be part of how frog eggs react to sperm entry or gravity in deciding which end should form the head?
One idea is to think of Wnt, etc. as analogous to transistors and capacitors etc. that you can find in TVs and air-conditioners and computers etc. which might lead one to conclude that these all work by the same mechanism. At some level, that would be true; at other levels false.
2) Arthropod embryos don't cleave into separate cells until the 13th mitotic cycle (~6-thousand cell stage). Until then they are syncytial, so that even fairly large proteins like transcription factors are free to diffuse around.
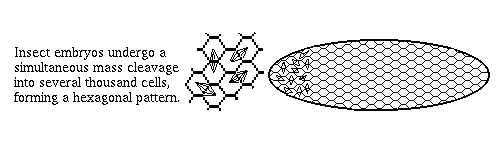
This is very different from vertebrates, and the great majority of phyla, in which nuclei are put in separate cells soon after fertilization, and before many decisions are made. Even small proteins can't diffuse from cell to cell.
3) Metamorphosis: Fly larvae shed their skins twice ("molts"),
and then during the third molt they pupate like caterpillars,
and the outer surface of the fly body is made of outpocketings
that had previously been undifferentiated sacs folded into the grub body wall.
"Imaginal Discs" Metamorphosis does not occur in many insects.
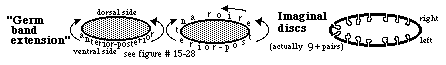
4) Most of the oocyte cytoplasm is produced in 15 mitotic sister cells, called nurse cells. These are not at all the same as follicle cells, although follicle cells perform a nursing function, and insects have follicle cells in addition to nurse cells. Vertebrates have follicle cells, but not nurse cells.
5) Mesoderm is internalized along the bottom, in an invagination process that looks like neurulation in vertebrates.
Endoderm folds inward from both ends toward the middle, In a process separate from the internalization of mesoderm.
Neural ectoderm is internalized by many separate cells, ingression over broad areas of the lower surface.
6) The ventral side is the center of embryological movements and differentiation, instead of the dorsal side as in vertebrates.
There is a very old theory, now supported by some new molecular evidence That arthropods evolved as upside-down equivalents of vertebrates, Or that vertebrates evolved upside-down, relative to arthropods.
Heart on the top; Nerve cord on the bottom!
Pipes carrying air from holes in the side, directly to each tissue!