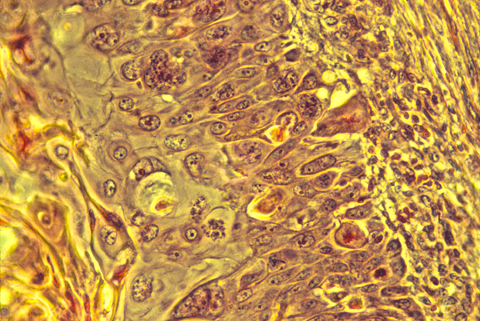
Embryology Biology 441 Spring 2010 Albert Harris
Cancer, from an Embryological Point of View
It is normal for body cells to grow and divide, and to crawl from place to place.
But cell growth and cell locomotion are normally controlled, by embryological mechanisms, which are only partly understood.
"Contact inhibition" means the normal inhibition of cell growth by crowding, and directional inhibition of cell crawling where once cell touches another.
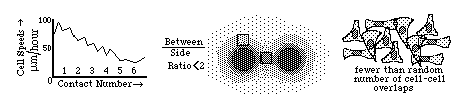
Diagrams of the quantitative experimental tissue culture measurements done by Michael Abercrombie and Joan Heaysman, by which they discovered and proved the existence of contact inhibition of cell locomotion (crawling). By these criteria, they also discovered that cancer cells in tissue culture have less sensitivity to contact inhibition; that seems likely to be at least part of the reason for their increased invasiveness. Other researchers later used the phrase "contact inhibition" to mean reduced rates of cell growth and mitosis in crowded tissue cultures. Cancer cells are likewise less inhibited than equivalent normal cells.
For many years, other scientists confused these two uses of the phrase "contact inhibition" (inhibition of growth as well as locomotion), assumed the same mechanism caused both, and that cancer cells always lacked both (which Abercrombie never claimed). Then people over-reacted in the opposite direction. The inhibition of locomotion now seems to be caused by prevention of actin fiber assembly near where cells touch each other. Much more research is needed to find out whether cancer cells are more able to continue crawling where they touch other cells, whether this is related to their uncontrolled growth, or if such abnormalities can be targeted by new kinds of chemotherapy.
These mechanisms of inhibition of growth and of locomotion may or may not have the same (or overlapping) mechanisms.
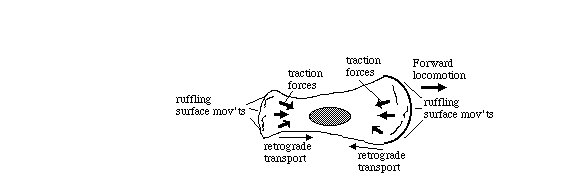
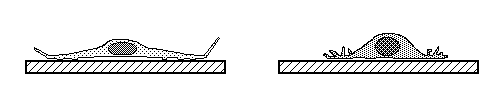
Tissue cells (including body cells in tissue culture) crawl by means
of traction forces exerted behind their leading edges. Traction
forces are exerted mostly behind areas where the plasma membrane
bends, bubbles and fold irregularly in a process called "ruffling"
(as can be seen in the video time lapse sequences below).
These cell surface movements were was once thought to be peristaltic, but
now are now known to be caused by continual re-assembly of cytoplasmic actin.
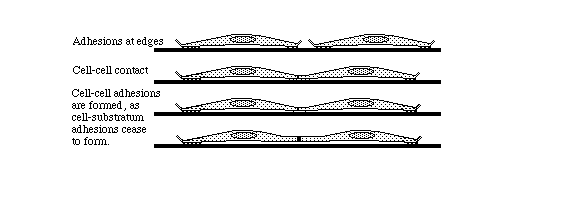
Part of contact inhibition is the paralysis of ruffling where two cells touch each other,
as shown in the diagram below.
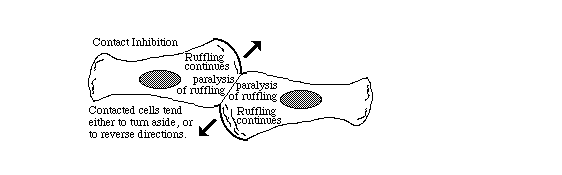
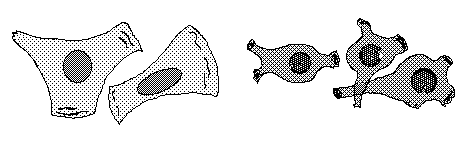
Many tissue cells behave as if actin assembly is somehow turned off where one cell touches another. Such cells behave as if the mechanism of locomotion is inhibited next to cell-cell adhesions. Sometimes one or both cells turns or crawls away from the cell-cell contant; and sometimes new cell-cell adhesions form, and the adhering parts of both cell margins cease locomotion and ruffling.
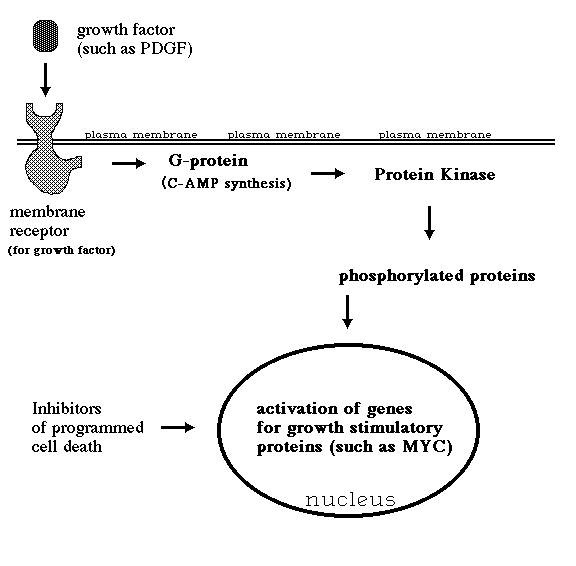
II) The great majority (>95%) of human cancers are caused by somatic mutations in a few specific genes (called "Oncogenes").
In many other species, large fractions of cancers are caused by communicable viruses. (examples include cats and turkeys)
This department gives a very good course specifically about oncogenes and how they cause cancer.
III) Several sexually transmitted papilloma viruses cause cervical cancer in women. A vaccine has recently been developed which inhibits infection by these cancer-causing papilloma viruses.
Many newspapers etc. have confused this with 'a vaccine against cancer.'
An editorial in the News and Observer "explained" that the vaccine "Is not yet approved for boys." but doesn't ask "Why not?".
These viruses infect both sexes: but the cancers they cause are in females.
What is their reasoning for vaccinating only one sex? After all, from whom do females catch these viruses?
IV) Cancer cells retain many of the properties of whichever differentiated cell type they began from: