Lecture 2, September 2: A chemical foundation for biology
First Lecture:
Please memorize the chemical structures of the chemicals below:
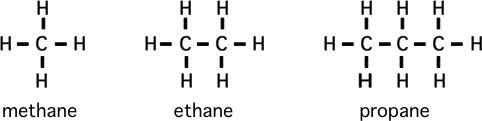
(longer ones are butane, pentane, .... octane)
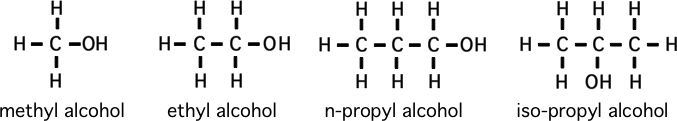
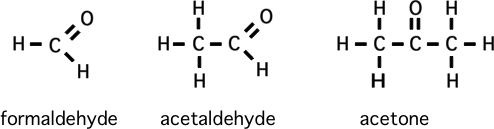
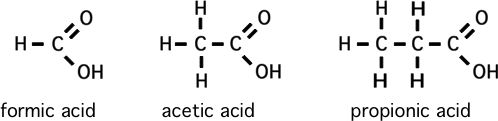
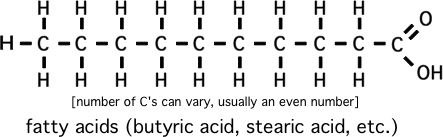
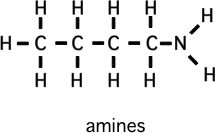
How to predict relative boiling points (will it be a gas?)
& predict melting points (liquid or solid at body temp.?)
Predicting solubilities in water? or in oil? ("lipids")
1) Bigger molecules are more liquid, and ones that are bigger than that are more solidThe concept of isomers (as in the cases of propyl alcohol and acetone)
(other properties being equal, like binding between molecules)2) Alcohol, acid and amino groups bond to each other, thereby making molecules behave as if bigger. (bigger, heavier, more liquid, or even solid)
3) "Like dissolves like" (chemicals dissolve in water if parts of them bond to water) (when a chemical has NO such bonding groups, the chemical may dissolve in oils, but NOT dissolve in water)
amino acids
Guess how are "alpha amino acids" different from, say, beta amino acids? (can the alphas beat the betas in a fight, or something like that?) (hint: no)
Please memorize the chemical structures of the following amino acids:
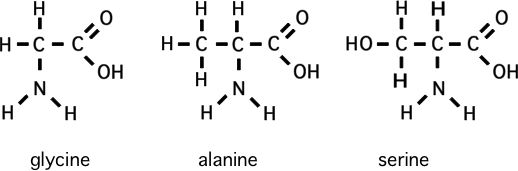
But don't bother to learn aspartic acid..lysine etc.
Proteins are chains of 20 different kinds of alpha amino acids
Questions for chapter two: (To expect on the exams.)
1) Formaldehyde is to methane as what is to propane? (structurally)
*2) Acetic acid is to what
as ethane is to gasoline, kerosene, & fuel oils?
3) What chemical would you get if you added a methyl group to the alpha carbon of glycine?
4) What chemical would you get if you added a hydroxy to a certain part of alanine? (where could you add a hydroxy to an alanine?)
* 5) Guess the structure of beta alanine!
Contrast this with alpha alanine
(the structure that you learned!)
6) What would you guess is the one amino acid that cannot have a beta isomer? (hint: it's easy!)
*7) Figure out how many different isomers there are of butyl alcohol. **Pentyl alcohol (*** figure out the general equation for numbers of isomers, if "n" is the number of carbons)
8) What are the patterns of differences in
a) melting and boiling temperatures?
b) solubility in water?
c) solubility in oils?
*first: depending on number of carbons in the chain?
**second: comparing ethane to ethyl alcohol to acetic acid, etc. (I admit that formaldehyde is a gas)
9) Imagine molecules in which one end would dissolve in water, but the other end would NOT! Sketch!
*10) Would you expect it to be possible to have a 3-carbon compound, with OHs on all carbons?
* Why would this chemical be gooey? and taste sweet? (please don't go around tasting chemicals, however!)